We are a campfire family. There isn't much we'd rather be doing on an evening then hanging out around a campfire. There's just something so relaxing and meditative about watching the flames or, even better, roasting something on them. In fact, one of my favourite presents this past year was a
s'mores roaster - that's right - the WHOLE s'more gets toasted, not just the marshmallow, made exceptionally better by the use of dark chocolate and occasionally the addition of peanut or almond butter. No one can deny that fire is pretty awesome and people and fire go wayyy back. Plus there is the ever exciting "adding a log" or "poking with a stick" - a good fire pokey stick is intrinsically important. We don't really care where the fire is - park, shoreline, backyard - anytime is fire time (except during fire bans obviously).
 |
| Fire + Food + Goofy Children = Awesome family time |
We've taught Beans and Bunny from a very early age all about fires and fire safety. The important rules like no running near the fire, keep a bucket of water nearby, don't leave a fire alone (they get lonely) and don't put your marshmallow directly in the flame. It was one of those rules that prompted this post - the bucket of water. It wasn't the why of it, they know to keep it there in case the fire jumps out of it's little circle and for end of the night dousing. It was the how - how does the water stop the fire?
 |
| It is hard to take good campfire pictures at night with a phone |
The answer to this one is pretty simple so to make it a nice, well rounded post I'll also put in assorted random campfire pictures of us or food or us with food.
 |
| Who doesn't love cooking stuff on a stick - SPIDER DOG! |
To answer the question we first have to know how fire works. A campfire is an exothermic reaction involving the release of energy rather than endothermic, one that sucks in energy, which is why it has a lovely glow and toasts things. The burning of wood is combustion - a rapid oxidation reaction that releases energy in the form of heat and light. To catch things on fire you need three things - fuel/combustible material (wood), and oxidizer (oxygen) and a heat source that gets it past the flash point of your fuel (lighter - which does it's own mini reaction with a gas, oxygen and a flint spark). Once lit the whole thing continues in a chain reaction and it sustains itself by it's own release of the heat and with a continuous supply of fuel and oxygen it will keep going (the aforementioned "adding a log" fun).
 |
| Behold! I have combined wood, oxygen and heat! Muahahaha! |
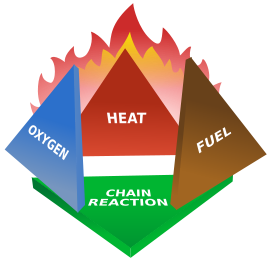 |
| Stuff fire needs |
To stop fire you need to take away at least one of the things it needs. Water takes away two - it stops the fire from being able to access the free oxygen in the air by displacing it and it turns to steam (usually quite dramatically), which carries the heat away from the fire and lowers the temperature back down so it no longer burns. Even though
water has oxygen in it, it is bonded to the hydrogen and not accessible for the combustion process.
Cool Fact: Astronauts can't have campfires because the process depends on gravity to constantly supply the fire with oxygen. Without gravity the fire will quickly have only have only it's own combustion products surrounding it and extinguish itself.
 |
| Astronauts cannot have this fantasticness. |
And now, as promised, more pictures of campfires:
 |
| A nice Georgian Bay shoreline fire |
 |
| Cooking stuff on a stick is good, but this is a definite step up! |










No comments:
Post a Comment