It’s the deceptively simple questions that always trip you up.
It rained yesterday. I would have been happy to explain the water cycle or re-answer Beans’ question on the composition of clouds for the fourteenth time (water not fluff – she remains unconvinced) or even take a stab at how plants use the water. At 7:45am what I was unprepared to answer was Bunny’s question.
What is water made of?
It started off okay – I know what water is made of. It’s made of hydrogen and oxygen. Two hydrogen atoms and an oxygen atom. H2O. She knows what oxygen is. She does not know what hydrogen is or what an atom is or why two things in air hook up and become liquid. Or how they find each other. Or how they stick together. I used to know. At 7:45am on a Tuesday morning I do not know.
I have one rule I made for myself when answering questions from the girls. Never make something up. Admit you don’t know and then find out together.
So here’s what we found. I am hoping it will help the next parent whose kid isn’t satisfied with “H2O” as an answer.
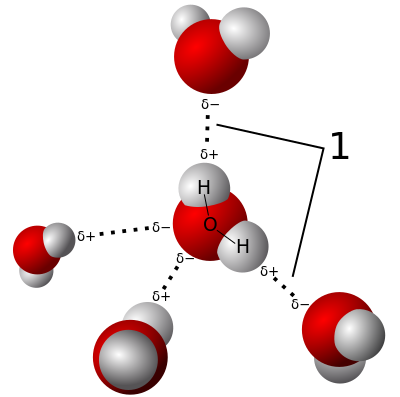
A single drop of water has millions of water molecules in it. Water molecules each have one oxygen atom (red) and two hydrogen atoms (white) connected by covalent bonds. Covalent just means they share electrons to stick together. Hydrogen atoms are positively charged and oxygen atoms are negatively charged when stuck together (dipole).
I think it’s kinda cute. I mean slap some googly eyes on that guy and it could happily star in a morning cartoon.
But then how do they stick together to make water? That would be hydrogen bonding. When lots of water molecules hang out together the electrical attraction from the charges make them get really close together, making them hard to get apart which raises the boiling point and makes it a liquid at room temperature. They are also constantly breaking apart and sticking back together, which give water some of its more unique qualities.
Water molecules stickin’ together. Bunny and I think the water molecules look like cartoon frog heads.
Here we are enjoying the properties of hydrogen bonding on our vacation:
Some cool water things to know:
- Water is 11.1% hydrogen and 88.9% oxygen by mass even though there are two hydrogens. This is because hydrogens are way smaller atoms than oxygens.
- Water is the only natural thing on earth that can be in all three states: liquid, solid and gas.
- The water that is on earth now is made up of the same exact elements that made the water on the planet when dinosaurs were here.
- Trees are two thirds water. So is your brain.
PHEW! Well, now we will all have a big glass of hydrogen covalently bonded with oxygen. I’m going to put a tea bag in mine.
References:


No comments:
Post a Comment